Download PDF format
Russian Journal of Parasitology, 2017, V. 40, Iss.2
DOI:
Received 19.01.2017
Accepted 06.04.2017
MONITORING OF TRICHINOSIS IN PIGS AND WILD BOARS IN TERMS OF HAZARDS TO HUMAN HEALTH IN THE PROVINCE POMERANIAN – POLAND
Balicka-Ramisz A.1, Grupiński T.2, Laurans Ł.3, Ramisz A.1, Pilarczyk B.1, Udała J.1
1 Department of Biotechnology of Animal Reproduction and Environment Hygiene, West Pomerania University of Technology, 71-466 Szczecin, Poland, e-mail: abalicka52@gmail.com
2 District Veterinary Inspectorate, 71-337 Szczecin, Poland
3 Chair and Clinic of Infectious Diseases and Hepatology, Regional Hospital 70-204 Szczecin, Poland
Abstract
The main reservoirs of trichinosis in the province Pomeranian (Polish) are wild boars and pigs which are still a serious threat to human health. The aim of the study was to investigated the prevalence of Trichinella spp. among wild boars and pigs in province Pomerania. From the veterinary and epidemiological perspective it was of the prime importance to identify the reason for the increasing prevalence of Trichinella spp. larvae infections among wild boars observed in the years 2008‒2013. In the animal study of parasitology for Trichinella larvae presence were performed post mortem by digestion method. Evaluation of microscopic samples of muscle preceded by digestion tests in artificial stomachs. Trichinella diagnosis in humans was based on immunoassay for the detection of presence Trichinella specific antibodies in serum. The assessment was based on official data on the number of cases of trichinosis in pigs and wild boars, derived from the annual reports of the Veterinary Inspectorate in Szczecin and annual bulletins of the National Institute of Public Health ‒ National Institute of Hygiene. In 2008, 16 583 tested boars Trichinella larvae were found in 92 cases, which accounted for 0,55 %. However, in 2013 it was 158 infected animals, which constituted the prevalence of 1,19 %. In 2005, 2006 and 2007 on Polish territory were major outbreaks of epidemic trichinosis. The largest of these took place in the province West Pomerania, where around 300 people were hospitalized. The diagnosis of trichinosis is based on the clinical picture, an interview with epidemiological and laboratory diagnostic examinations. The conducted monitoring over the years has shown cyclical nature of the occurrence of a tendency to create an epidemic.
Keywords: trichinellosis, pigs, wild boars, epidemiology, West Pomerania Region Poland.
Introduction
In recent years implementing the provisions of the European Union in Poland made a number of significant changes for the sealing of meat inspection. Introduced restrictive provisions governing trade in meat slaughter and sanitary-veterinary examination of slaughter animals and meat and sanitary supervision over the production and marketing of food of animal origin (Flis, 2012). The laboratory diagnosis of trichinosis both methods, direct and indirect introduces a more sensitive assay. The method of etching and immunoassays substantially. They contributed to the detection of larvae Trichinella in animals. While the use of molecular techniques made it possible to determine the species or genotype of Trichinella isolates. The high ensitivity of PCR techniques, multiplex PCR and PCR-RLFP ‒ allows for genetic identification of the Trichinella based on the examination of a single larva. The indirect methods that are used in the diagnosis of the genus Trichinella in animals and humans are recognized also by indirect immunofluorescence, indirect hemagglutination method, competitive inhibition assay, immunoblotting and ELISA method, they have applications by routine testing in the diagnosis of trichinosis. PCR method and its variants are highly sensitive and specific, but their proper execution requires great skill (Pozio, La Rosa, 2003).
The only way to prevent trichinosis is the elimination from the market of animals infected with Trichinella. The method recommended by The ICT for testing meat for consumption in the direction of Trichinella larvae, at the stage of post-mortem, is the method of etching, which consists of a direct confirmation of the presence of larvae of Trichinella spp. In carcasses analyzed (Nöckler et al., 2000, Commission Regulation, 2005). A greater percentage of contracting trichinosis animals have been observed in countries with large forested areas. This situation is related to the interaction between animals and the environment in which they live.
In recent years conducted extensive research in different parts of the world of animals ‒ reservoirs of trichinosis. In Europe, main natural reservoirs animals are wild boars (Sus scrofa), foxes (Vulpes vulpes) and rodents. These are primarily carnivores or omnivores. The literature also found the presence of Trichinella in: wolves (Canis lupus), bears (Ursus arctos), jackals (Canis aureus), badger (Meles meles), European pine marten (Martes martes), otter (Lutra lutra), raccoon dogs (Nyctereutes procyonoides) (Pozio et al., 2009, Pannwitz et al., 2010).
The spread of trichinosis importance may also have dogs, cats. Due to the large migration of people with different cultural habits, or in countries where there is a tradition of eating dog meat, the meat may pose some risk to human health. In the sixties observed a high prevalence of Trichinella infection in dogs in Greenland, Alaska, which probably resulted from the feeding of meat of hunted animals (Madsen, 1961). In Finland the incidence of trichinosis in animals forest is higher than in most other EU countries, which was echoed in the larger percentage of dogs trichinosis infection. This fact was explained in the Finnish way of life, where dogs often have access to dead animals free-living (Pozio, 1998). Since 1986 in the European Union slaughter of dogs and their meat trade is prohibited. In Russia, at 11,9 %, it was found infected people trichinosis after eating dog meat (Ozeretskovskaya, 2005).
In China was described two outbreaks, which were linked to the tradition of eating raw or parboiled dog meat. In this country, there is no obligation survey of dog meat for trichinosis [9]. In Egypt, the larvae of T. spiralis isolated from stray dogs (Wang et al., 2006).
Despite such significant changes in Poland as the risk for trichinosis caused by the invasion of T. spiralis (Mikhail, 1994; Cabaj et al., 2005).
The human biotope most important, from the point of view of the epidemiological has pigs. Despite the improvement of research methods, pork constantly comes to human infection with this parasite. The most common cause of poisoning is consumption of meat products prepared for its own use (Sadkowska‒Todys, Gołąb, 2009) .
Materials and methods
The degree of infection significantly influenced by local environmental conditions. In the analysis of epizootic situation in Poland, West Pomerania (North 54 ° 34'10 "N, 52 ° 37'28 noon" N, 14 ° 07'22 west "E, east of 16 ° 58'55" E) is considered too the region with the most intense invasion of trichinosis. This is due to the large (more than 35 % of all sites) afforestation of the area and a large concentration of various water bodies (Fig. 1)
Fig. 1. Map province West Pomerania in Poland (central Europe)
The aim of the study was to present the epizootic situation of trichinosis province Pomeranian in pigs and wild boars, which are primary reservoirs of Trichinella. In the animal study of parasitology for Trichinella larvae presence were performed post mortem by digestion method. Evaluation of microscopic samples of muscle preceded by digestion tests in artificial stomachs. Trichinella diagnosis in humans was based on immunoassay for the detection of presence Trichinella specific antibodies in serum.
The assessment was based on official data on the number of cases of trichinosis in pigs and wild boars, derived from the annual reports of the Veterinary Inspectorate in Szczecin and annual bulletins of the National Institute of Public Health ‒ National Institute of Hygiene.
Results and discussion
In the area of West Pomerania are conducted for a long period of time researching the prevalence of Trichinella larvae in the wild animals.
In the nineties Trichinella larvae were found in 0,18 % from 4,57 % wild boar and foxes (Ramisz et al., 1998; Ramisz et al., 2001). In 2006‒2009 was diagnosed with trichinosis in wild boars 0,64 % [16]. Analyzing the period 2008‒2013 further notice growth has Trichinella infected animals (Table 1). In 2008, 16 583 tested boars Trichinella larvae were found in 92 cases, which accounted for 0,55 %. However, in 2013 it was 158 infected animals, which constituted the prevalence of 1,19 %. Overall, IP were surveyed during on 51 936 wild boars degree of infection was 0,77 %.
Table 1
The presence of Trichinella spiralis in pigs and wild boars in the province West Pomerania in 2008‒2013
|
Years |
Pigs |
Wild boars |
||||
|
Number of respondents |
Number infected |
% |
Number of respondents |
Number infected |
% |
|
|
2008 |
1 077435 |
5 |
0,00046 |
16 583 |
92 |
0,55 |
|
2009 |
872823 |
6 |
0,00068 |
15 540 |
110 |
0,71 |
|
2010 |
1 092 885 |
2 |
0,00018 |
13 105 |
112 |
0,85 |
|
2011 |
968 902 |
0 |
0 |
6 708 |
83 |
1,24 |
|
2012 |
988 151 |
1 |
0,000101199 |
16737 |
142 |
0,8484 |
|
2013 |
2177545 |
4 |
0,000184 |
13233 |
158 |
1,1939 |
|
Total |
4 012045 |
13 |
0,000324 |
51 936 |
397 |
0,77 |
It is increased in contagion trichinosis in wild boars Western Pomerania which may also have to explain the actions taken by humans. In the second half of the nineties in the Polish Western (along the Oder), including in the province West Pomeranian undertaken an extensive campaign of mass oral vaccination of foxes against rabies. The results of vaccination proved to be very good, because in more than 70 % of foxes found antibodies against the rabies virus (research conducted at the National Veterinary Institute in Pulawy). The consequence of vaccination was carried out by a significant increase in the number of foxes Western Pomerania. Trichinosis was found in 4,35 % tested foxes. Rabies was a biological factor regulating the population of foxes in the biotope. Also decreased interest in the skins of foxes and hunted animals are often left in the environment and can be a source of infection of wild boars Trichinella. The high prevalence of infection in animals Trichinella larvae impact on the number of cases of people with the disease.
Trichinosis despite an increased action on health care remains a threat to human health. In 2005, 2006 and 2007 on Polish territory were major outbreaks of epidemic trichinosis. The largest of these took place in the province West Pomerania, where around 300 people were hospitalized (Fig. 2). The diagnosis of trichinosis is based on the clinical picture, an interview with epidemiological and laboratory diagnostic examinations. No deaths were recorded. The conducted monitoring over the years has shown cyclical nature of the occurrence of a tendency to create an epidemic.
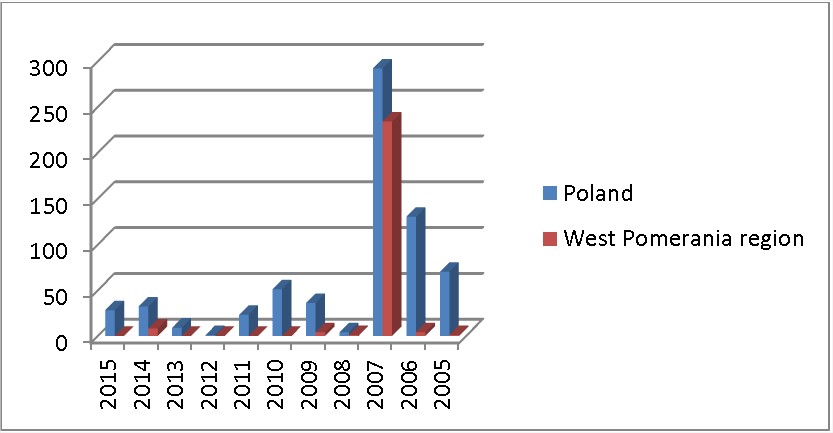
Fig. 2. Number of cases of trichinosis people in 2005‒2014 in West Pomerania
The analysis of global data shows that in 1986‒2009 the main source of trichinosis infection in human are domestic pigs (53 %). However in period 2010‒2013 in majority of cases the source of infection was a meat from wild animals (54 %) (Murrell, Pozio, 2009). In UE, more cases from 2013 was coming from pork, mostly in this incidents took place in eastern Europe. On trichinosis frequency have influence a social-economic disruptions that change a pork production practice (Djordjevic et al., 2003). This fact have influence on changeability of trichinosis infection on Ukraine. In 3 regions (Zakarpacie, Żytomski, Zaporoże) wild predators were infected in 30‒50 %. Infection was shown in wild boars 3 %, wolfs 15 %, foxes 10 %, badgers 5 % and martens 2 % (Akimow, Didik, 2009). Trichinosis was able to spread among animals by increasing number of raccoon dogs, that are eating mainly carcass. Research that was carried on in Germany shows, that the most infected animals are living in the woods: wild boars, foxes and for short raccoon dogs. The result of meat analysis shows 0,003 % trichinosis infection in wild pig meat and < 1 % infection in red (Mayer‒Scholl et al., 2011). However in raccoon dogs the positive results were in 1,9 % of animals (Pannwitz et al., 2010). Increasing of infection extensity leads to spreading of trichinosis in nature. However we can’t ignore a domestic animals as a source of trichinella infection. New trend in pigs breeding, specially more ecologic productions and free breeding can increase the risk of trichinosis (Murrell, 2016). In 2014 the research carried in Romania shows the highest rate of trichinella infection in bears (12,93 %) and wild boars (1,66 %). Domestic pigs were infected in 0,20 % (Nicorescu et al., 2015). In Poland the level of infections since 1968 is 0,002‒0,005 %. However is observed a systematic increase of infection in wild boars from 0,11 % in 1964 to 0,39 % in 1992.
In spreading of trichinosis we can’t ignore other animals like horses or coypu (Wieteska, 2012). The criteria of UE regulate a removal of shouted animals from hunting area (Regulation No 1774/2002). Eating an infected meat, that was not examined is the main reason that people can get sick on trichinosis. The wild boars are potential source of infection for people in Poland.
The main reservoirs of trichinosis in Poland are still wild boars and pigs. But you cannot ignore other animals as a source of potential danger of Trichinella people.
Acknowledgments
The financial support and facilities provided by Department of Biotechnology of Animal Reproduction and Environment Hygiene, West Pomerania University of Technology, Szczecin, Poland District Veterinary Inspectorate, Szczecin, Poland, Szczecin, Poland Chair and Clinic of Infectious Diseases and Hepatology, Regional Hospital Szczecin, Poland.
References
1. Akimow I. A., Didik J. M. The spread of Trichinella (nematode, trichinellidae) among wild and domestic animals in different regions of Ukraine abstract XIVconference of Ukrainion Scientific Society of Parasitologists Uzhorod 21‒24 September, 2009, p. 6.
2. Cabaj W., Moskwa B., Pastusika K., Bień J. Distribution of trichinella species in Poland. Kosmos, 2005, vol. 54, pp. 95‒103.
3. Djordjevic M., Bacic M., Petricevic M., Cuperlovic K., Malakauskas A. C. M., Kapel K. D., Murrell K. D. Social, political and economic factors responsible for the reemergence of trichinellosis in Serbia: a case study. J. Parasitol., 2003, vol. 89, pp. 226–231.
4. Flis M. The epizootic and epidemiological situation of trichinellosis in Poland in 2010. Życie Weterynaryjne, 2012, vol. 877, pp. 286‒288.
5. Mayer‒Scholl A., Reckinger S., Nöckler K. The sylvatic Trichinella cycle and its implications for Trichinella control in Germany Berl. Munch. Tierarztl. Wochenschr., 2011, vol. 124, pp. 450–456.
6. Murrell K. D., Pozio E. Worldwide occurrence and impact of human trichinellosis, 1986–2009. Emerg. Infect. Dis., 2011, vol. 12, pp. 2194–2202.
7. Murrell K. D. The dynamics of Trichinella spiralis epidemiology: Out to pasture? Vet. Parasitol., 2016, vol. 15, pp. 92‒96.
8. Madsen H. The distribution of Trichinella spiralis in sledge dogs and wild mammals in Greenland under a global aspect. Meddelelser on Grønland, C. A. Reitzels Forlag, Bianco Lunos Bogtrykkeri A/S København., 1961, 1, p. 124.
9. Mikhail E. M., Mansour N. S., Awadalla H. N. Identification of Trichinella isolates from naturally infected stray dogs in Egypt. J. Parasitol., 1994, vol. 801, pp. 151‒154.
10. Nicorescu I. M. D., Ionita M., Ciupescu L., Buzatu C. V., Tanasuica R., Liviu M. New insights into the molecular epidemiology of Trichinella infection in domestic, pigs, wild boars and bears in Romania. Vet. Parasitol., 2015, vol. 1, pp. 257–261.
11. Nöckler K., Pozio E., Voigt W. P., Heidrich J. Detection of Trichinella infection in food animals. Vet. Parasitol., 2000, vol. 93, pp. 335–350.
12. Ozeretskovskaya N. N., Mikhailova L. G., Sabgaida T. P., Dovgalev A. S. New trends and clinical patterns of human trichinellosis in Russia at the beginning of the XXI century. Vet. Parasitol., 2005, vol. 132(1‒2), pp. 167‒171.
13. Pannwitz G., Mayer‒Scholl A., Balicka‒Ramisz A., Nöckler K. Increased Prevalence of Trichinella spp., Northeastern Germany, 2008. Emerg. Infect. Dis., 2010, vol. 16(6), pp. 936‒942.
14. Pozio E., La Rosa G. PCR-derived methods for the identification of Trichinella parasites from animal and human samples. Methods Mol. Biol., 2003, vol. 216, pp. 299–309.
15. Pozio E., Rinaldi L., Marucci G., Musella V., Galati F., Cringoli G., Boireau P., La Rosa G. Hosts and habitats of Trichinella spiralis and Trichinella britovi in Europe. Int. J. Parasitol., 2009, vol. 39, pp. 71–79.
16. Pozio E. Trichinellosis in the European Union: Epidemiology, ecology and economic impact. Parasitol. Today, 1996, vol. 14, pp. 35–38.
17. Ramisz A., Balicka‒Ramisz A., Bieńko R., Grupiński T. The prevalence of Trichinella sp. in foxes in the Western Poland. Medycyna Wet., 1998, vol. 54, pp. 747‒749.
18. Ramisz A., Szymborski J., Balicka‒Ramisz A. Epidemiological studies on trichinellosis among swine, wild boars and human in Poland. Parasite, 2001, vol. 8, pp. 90‒91.
19. Ramisz A., Grupiński T., Balicka‒Ramisz A., Udała J., Laurans Ł. Prevalence of Trichinella sp. in red foxes and wild boars in the Western Pomerania region. Bull. Vet. Inst., 2001, vol. 55, pp. 199‒201.
20. References Commission Regulation (EC) No 2075/2005 of 5 December 2005 laying down specific rules on official controls for Trichinella in meat. Official J. European Union, 2005, vol. 338, pp. 60–82.
21. Regulation No 1774/2002 of the European Parliament and the Council of Europe. Sadkowska‒Todys V., Gołąb M. Trichinellosis in Poland in 2007 (Włośnica w Polsce w 2007 roku). Przegl Epidemiol., 2009, vol. 63, pp. 263‒266.
22. Wang Z. Q., Cuia J., Xu B. L. The epidemiology of human trichinellosis in China during 2000–2003. Acta Tropica, 2006, vol. 97, pp. 247–251.
23. Wieteska S. Hazards caused by some wild animals, reptiles, insects in the polish territorial area. Acta Universitatis Lodziensis, 2012, vol. 274, pp. 141‒152.
© 2017 The Author(s). Published by All-Russian Scientific Research Institute of Fundamental and Applied Parasitology of Animals and Plants named after K.I. Skryabin. This is an open access article under the Agreement of 02.07.2014 (Russian Science Citation Index (RSCI) http://elibrary.ru/projects/citation/cit_index. asp) and the Agreement of 12.06.2014 (CABI.org / Human Sciences section: http://www.cabi.org/Uploads/ CABI/publishing/fulltext-products/cabi-fulltext-material-from-journals-by-subject-area.pdf).