Link t full russian text
Download PDF format
Russian Journal of Parasitology, 2017, V. 40, Iss.2
DOI:
Received 23.02.2017
Accepted 11.06.2017
A CONTRIBUTION AND PATHOLOGY OF E.COLI AND PSEUDOMONAS AERUGINOSA BACTERIAL INFECTION ON SOFT SHELL TURTLE RAFETUS EUPHRATICUS (GRAY, 1864) EAST HAMMAR MARSHES, IRAQ
Majid A.A.Bannai, Essa T. Mohammad, Ahmed J. Shamary, Khassn A.Al- Najjar, Fawzi M. Al-Khwaja, Mohammed A. Aldoghachi
Marine Vertebrate, Marine Biology, Marine Science Center, Basra University, Iraq.Corresponding author email: Majidbannai65@gmail.com[i]
Abstract
E.coli and P. aeruginosa bacterial infection on Soft shell turtle Rafetus euphraticus. Isolation and identification diagnosis which that on these species affected, biochemical test and clinical signs. Samples of the Soft shell turtle Rafetus euphraticus Gray, 1864 were collected from East Hammar marshes during summer season of the year 2016 .The infection isolated with a percent of 40%. Clinical Finding obvious that the incidence of E.coli and P. aeruginosa infection. Some of Soft shell turtle show clinical abnormalities with E.coli and P. aeruginosa. The most common clinical signs were external haemorrhage, Histopathological changes revealed degeneration and necrosis in all organs associated with Chronic inflammatory cell infiltration and melanomacrophage cells were detected in all turtle tissues. This study showed that P.aeruginosa infection is common in the Soft shell turtle Rafetus euphraticus. So, this study was designed to make a survey of bacterial infestation of Soft shell turtle Rafetus euphraticus Gray, 1864 East Hammar marshes during the summer of 2016.
Key words: E.coli, Pseudomonas aeruginosa, bacterial infection, Soft shell turtle Rafetus euphraticus Gray, 1864, East Hammar marshes, Iraq
Introduction
The Euphrates soft shell turtle Rafetus euphraticus is classified as Endangered on the IUCN Red List and is thought to have undergone large, recent population declines, Species information in Iraq is limited to a few rapid surveys with little detailed information on breeding and distribution (Nadheer et al., 2015). Some of literature mentioned Global range and of distribution occurs in the Euphrates and Tigris rivers, from south-eastern Turkey, through Syria and Iraq to the Arabian Gulf extending into southwestern Iran. It occurs from elevations of 1000 m to sea level (Baran & Atathur, 1998). The range in Iran is limited to central and western Khuzestan province and related to the main rivers. The border areas of Iran and Iraq contain many important rivers considered as the “habitats” of R. euphraticus including Karkheh, Dez, Karoon, Djarrahi, Shahoor and Bahmanshir. Other important habitats are the wetland sites listed as “international wetlands (Ramsar Site) like Shadegan and Hour-Al Azim (Asghar and Mola, 2011).
All populations of organisms, including aquatic animals is limited partly or wholly in the ecosystem (Real, 1996). The prevalence of the disease in the ecosystem is affected by many environmental factors, including infectious and stressors (Nils Kautsky et al., 2000). Bacteria are everywhere, and occur in most freshwater environments, can be found in the water column and in the sediment (Hazen, 1979). A bacterial are adapted to environments that have a wide range of conductivity, turbidity, pH, salinity and temperature (Hazen et al., 1978). Optimum temperature may depend on the particular strain is under investigation, but generally range from 25℃ to 35 degrees Celsius (Meyer, 1970).
Pseudomonas spp. are widely spread in natural sources of water and associated with septicemia in aquatic animals (Roberts, 2001). These bacteria are considered opportunistic pathogens, causing disease when the host is subjected to stress. A number of aquatic animals including fish, frogs and soft-shelled turtles are reported to be susceptible to Pseudomonas spp. with moderate to high losses (Somsiri and Soontornvit, 2002). The etiological agents commonly found are P. diminuta, P. fluorescens, P. putida and P. aeruginosa with different degrees of virulence. The characteristic symptom of the disease produced by Pseudomonas bacteria is a remarkable septicemia hemorrhage in the skin of the mouth region, opercula and ventral side of the body (Wakabayashi and Egusa, 1972).
Conventional microbiological methods needed to identify bacteria from fish are often limited by the length of time required to complete the assays. In recent years, PCR have overcome problems associated with culture- based techniques, enabling the detection of bacteria directly in clinical samples without the need for previous culturing (Gonzalez et al., 2004). Escherichia coli is a commensal bacterium lives harmless in intestinal microflora in variety of animals including man, however sometimes they cause fatal diseases in humans, mammals and birds (Belanger et al., 2011) Based on pathogenicity and site of infection E. coli strains are classified into three distinct groups such as commensal strains, intestinal pathogenic strains, and Extra intestinal pathogenic E. coli (ExPEC) strains (Lyhs et al., 2012)
So, this study was designed to make a survey of bacterial infestation of Soft shell turtle Rafetus euphraticus in the marshes
Materials and Methods
A total of 15 Soft shell turtle R. euphraticus were collected from East Hammar marshes during the summer of 2016; the infected turtle were acclimatized in fresh water of glass aquarium in the laboratory conditions approximately at 25°C. The turtle were allowed to feed on commercial fish-food daily and were regularly monitored whether any death or ulcerative symptoms occurred. The bacteria isolates and culture from affected organs by a sterile loop and streaked on the pre-prepared sterilized nutrient agar. Media were subjected to various tests beginning from the study of their growth morphology on different agar media to different microbiological identification tests such as gram staining and biochemical identification tests such as oxidase, methyl red, Vogesproskeur, urase, H2S, Triple sugar iron, indol. A sterile wire loop was used to collect a loop full of each undiluted and inoculated on the surface of nutrient agar, MacConkey agar and Pseudomonas base agar to obtain pure cultures as well as study their morphological characteristics. The agar plates were incubated at 37°C for 24 h for appropriate colony formation.
The predominant bacterial colonies from the media were isolated, purified and characterized following standard methods (Sneath, 1986; Lacey, 1997; Pelczar, 1957).
Results
Isolation and identification diagnosis of E.coli and Pseudomonas aeruginosa bacterial infection on Soft shell turtle Rafetus euphraticus can be based on these species are affected, biochemical test and clinical signs of the disease. The biochemical test of E.colai and Pseudomonas aeruginosa were negative to test urease, coagulase and Voges–proskauer. Morphology of colonies of Pseudomonas aeruginosa are sticky ,shiny, slanted to green yellow color with thick growth of spiky do not contain whereas the E.coli are convex spherical colonies that are depended on the color of the colony media MacConkey agar where rosy color and is non –composed of the spores (table 1). The infection isolated with a percent of 40%. Clinical Finding study found that the incidence of E.coli and Pseudomonas aeruginosa infection. Some of Soft shell turtle show clinical abnormalities of E.coli and Pseudomonas aeruginosa were isolated and identified, and all of the collected Soft shell turtle showed one or more from the following signs according to the stage of disease; darkness in the color of the skin, Skin showed vacuolar degeneration and necrosis in the epidermal cells with mononuclear inflammatory cells infiltration in between the epidermal cells, detachment of the scales, large irregular hemorrhages on the body surface, Ulcerative on the skin varied from shallow to deep necrotizing ulcers, inflamed vent, exophthalmia, abdominal distension with sero-hemorrhagic fluids exuded from the vent as shown in Figure 2. The most common sign of disease, occurring in 2 of turtles examined. The dorsum of the neck flippers and tail, Eye lesions took several forms: a yellow deposit on the cornea adjacent to the eyelids indicated keratocon- junctivitis. Diseases of the skeletal system were uncommon. Ulcerative shell disease was characterized by the appearance of dark, focal discolorations on the carapace of farmed turtles. These progressed into pits or ulcers.
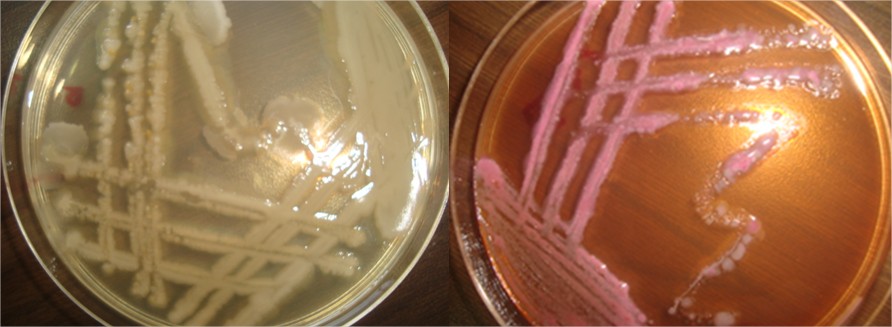
Fig (1) culture of the Pseudomonas aeruginosa and E.colai of bacterial infection
Table (1) Biochemical tests of contribution of E.colai and Pseudomonas aeruginosa bacterial infection on Soft shell turtle Rafetus euphraticus
|
Biochemical test |
Cramstin |
Oxidase |
Mir |
Vp |
Urase |
H2s |
Citrate |
Triple suger iron |
Indol |
Motality |
Coagulase |
|
E.coli |
- |
- |
- |
- |
- |
- |
- |
A/A |
+ |
+ |
- |
|
Pseudomonas aeruginosa |
- |
+ |
+ |
- |
- |
- |
+ |
A/K |
- |
+ |
- |

Fig (2) Soft shell turtle Rafetus euphraticus Gray, 1864 site of bacterial infection
DISSCUTION
Bacterial infections are a common cause of disease in turtles and tortoises. Proper nutrition, housing, and sanitation are the best methods of prevention ,however, even in the well-cared for turtles or tortoises, infections can still occur. The genus Pseudomonas is one of the most diverse bacterial genera, and its taxonomy has undergone many changes. During 2004, Pseudomonas spp. was isolated from Nile tilapia and African catfish (Clarias gariepinus), silver carp (Hypophthalmichthys molitrix) and grey mullet (Mugil cephalus) that were being reared in seventeen commercial fish farms in Kafr EI-Sheikh Governorate (Mesalhy, 2013). Bacteriological examinations of private fish farms in Kafr EI-Sheikh Governorate suffered from high mortalities, ranging from 17.6 to 22.9%. Revealed that 38 fish (36.9%) were infected with Pseudomonas fluorescens, 30 (29.1%) with Pseudomonas aureginosa, 19 (18.5%) with Pseudomonas anguilliseptica and 16 (15.5%) with Pseudomonas pseudoalkaligene (Masbouba, 2004). Pseudomonas infection has been incriminated as the most common bacterial infection among fish and appears to be stress related disease of freshwater fish especially under culture conditions (Kitao et al., 1993). Pseudomonas aerugenosa was isolated from skin, gills and stomach content of cultured Clarias gariepinus fingerlings in Nigeria (Oni et al., 2013). Data clearly indicates that infection is responsible for huge losses to aquaculture. Pseudomonas spp. Caused septicemia in aquatic animals (Roberts, 1978) and a number of aquatic animals including fish, frogs and soft-shelled turtles are reported to be susceptible to Pseudomonas spp. with moderate to high losses (Somsiri and Soontornvit, 2002). Pseudomonas spp. bacteria are considered as opportunistic indoor pathogens as their infection initiates an inflammatory response (Hirvonen et al., 2005; Huttunen et al., 2003).
Eissa et al. (2010) isolated different strains of Pseudomonas species namely Pseudomonas putida, P. aeruginosa, P. fluorescens and P. anguilliseptica from Oreochromis niloticus in Qaroun and Wadi-El-Rayan Lakes, Egypt, They reported that infected fishes showed irregular hemorrhages on body surface, especially at the ventral part of abdomen, eyes cloudiness; scales detachment and congested gills were observed. Internally, there were sanguineous fluids in the abdominal cavity of some fish. Bacteria invade the host tissue and cause infection and bacteremia in immune compromised hosts (HIV/AIDS, cystic fibrosis, bronchiectasis, severe chronic obstructive pulmonary disease, burns, malignancy or diabetes mellitus) (Feldman et al., 1998; Liu and Mercer, 1963). Furthermore extra intestinal pathogenic E. coli live as commensal in the intestines but sometimes they infect extraintestinal sites (Smith et al., 2007). It can cause various infections including gastroenteritis, urinary tract infection, meningitis and septicemia (Von BH and Marre R. 2005). E. coli is often considered an opportunistic pathogen (Hussain et al., 2014). Many strains of E. coli have specific virulence factors that provide them the inherent capability to cause disease (Hartl DL and Dykhuizen DE. 1984). E. coli is also a common contaminant of different food sources and water (Newell DG et al., 2011). And is frequently used as indicator organism to estimate fecal contamination in water.
The histopathological changes: Pseudomonas sp. could be considered as an opportunistic pathogen, which can survive on the surface of water or in the gut and may cause disease when unfavorable conditions developed (Kumaran et al., 2010).
Also, these results are in agreement with those of Hossain et al.(2006) and Musa et al.(2009). Similar lesions have been reported to occur in P.aeruginosa infected crabs gills where haemocytes are accumulated in the haemocoelic space. Necrosis is seen in most of the gill rachis. Epithelial lifting and Disrupted Pillar cells were also observed (Devakumar et al., 2013).
References
Asghar Mobaraki and Adel Mola ,2011 MESOPOTAMIAN SOFT SHELL TURTLE (Rafetus Euphraticus), THE STRANGEST TURTLE OF THE MIDDLE EAST Volume 5 • Issue 4 • March 2011 • ISSN 1990-8237
Baran & Atathur,( 1998).cited by( Asghar Mobaraki and Adel Mola ,2011 ).
Belanger L, Garenaux A, Harel JI, Boulianne M, Nadeau E, Dozois CM. 2011. Escherichia coli from Animal Reservoirs as Potential Source of Human Extraintestinal Pathogenic E. coli. FEMS Immunology and Medical Microbiology. 62: 1-10.
Bergey’s Manual of Systematic Bacteriology (2001). Vol. 2. Ed. N. R. Krieg and J.G. Holt. Williams and Wilkins Publishers, Baltimore.
Chesbrough,M.,2002.Medical Laboratory Manual for Tropical Countries. Butterwoths and Co. Ltd.,UK,pp:21-32.
Devakumar, D.; Jayanthi, J. and Ragunathan M.G.(2013):Herbal alternate to Pseudomonas aerginosa infection in a freshwater crab, OZIOTELPHUSA SENEX SENEX, International Journal of Biology, Pharmacy and Allied Sciences (IJBPAS), November, 2(11): 2142-2147.
Eissa. N.M.E. , Abou El-Ghiet. E.N. , A.A. Shaheen. A.A. and Abbass. A.2010: Characterization of Pseudomonas Species Isolated from Tilapia "Oreochromis niloticus" in Qaroun and Wadi-El-Rayan Lakes, Egypt . Global Veterinaria 5 (2): 116-121.
Feldman M, Bryan R, Rajan S, Scheffler L, Brunnert S, Tang H, Prince A (1998).Role of flagella in pathogenesis of Pseudomonas aeruginosa pulmonary infection. Infect. Immun. 66(1):43-51.
Gonza´lez Santiago F. ; Melissa J. Krug ; Michael E. Nielsen ;Ysabel Santos, and Douglas R. Call 2004.Simultaneous Detection of Marine Fish Pathogens by Using Multiplex PCR and a DNA Microarray. Journal of clinical microbiology, p. 1414–1419.
Hartl DL, Dykhuizen DE. 1984. The population genetics of Escherichia coli. Annual Reviews in Genetics. 1984;18:3168.
Hazen T. C., 1979, Ecology of Aeromonashydrophila in a South Carolina cooling reservoir.Microbial Ecology, 5: 179-195, http://dx.doi.org/10. 1007/BF02013525
Hazen T. C., Fliermans C. B., Hirsch R. P., and Esch G. W., 1978, Prevalence and distributionof Aeromonashydrophila in the USA, Journal of Applied and Environmental Microbiology, 36(5): 731-738
Hirvonen MR, Huttunen K, Roponen M (2005). Bacterial strains from moldy buildings are highly potent inducers of inflammatory and cytotoxic effects. Indoor Air 15(9):65-70.
Hossain,M.I., Farzana A.N., Hussain,M.A., Rahman,M.H and Satoru, S. (2006) : Disribution of Pseudomonas aeruginosa in swamps and its infection to Oreochromis niloticus. J. bio-sci. 14: 77-81.
Hussain T, Jamal M, Nighat F, Andleeb S. 2014. Broad Spectrum Antibiotics and Resistance in Non-target Bacteria: An Example from Tetracycline. Journal of Pure and Applied Microbiology. 8(4): 2667-2671.
Huttunen K, Hyvarinen A, Nevalainen A, Komulainen H, Hirvonen MR (2003).Production of proinflammatory mediators by indoor air bacteria and fungal spores in mouse and human cell lines. Environ. Health Perspect. 111:85-92.
Kitao, T.;Aoki T.;Fukudome, M.; Kawano, K.; Wada,Yo.; and Mizuno,Y.(1993): Serotyping of Vibrio anguillarum isolated from fresh water fish in Japan. journal of fish diseases , 6,175- 181.
Kumaran S.Æ B.; Deivasigamani Æ K. M.; Alagappan Æ M.; Sakthivel Æ S. and Guru Prasad (2010): "Isolation and characterization of Pseudomonas sp. KUMS3 from Asian sea bass (Latescalcarifer) with fin rot". Expert review of World J Microbiol Biotechnol, 26: (2): 359–363.
Lacey LA (1997). Manual of techniques in Insect pathology. Academic Press, New York, USA, p. 409.
Liu PV, Mercer CB (1963).Growth, toxigenicity and virulence of Pseudomonas Aeruginosa. J. Hygiene 61: 485-491.
Lyhs U, Ikonen I, Pohjanvirta T, Raninen K, Mنkela PP, Pelkonen S. 2012. Extraintestinal pathogenic Escherichia coli in Poultry Meat Products on the Finnish Retail Market. Acta Veterinaria Scandinavica. 54:64.
Masbouba, Imam M. (2004). Studies on Pseudomonas Infection in Fish in Kafr El - Sheikh Province. Unpublished M V Sc. Thesis, Tanta University.
Mesalhy ,S.A (2013) : A review of fish Diseases in The Egyptian Aquaculture . www.livestockfish.cgiar.org Musa,N., Wei, L.S. and Wendy, W. (2009): Bacterial diseases outbreak of African Catfish (Clarias gariepinus) from Manir River, Terengganu, Malaysia. J lif. Sci.,volume 3 no (5) :10-20.
Meyer F. P., 1970, Seasonal fluctuations in the incidence of disease on fish farms, in S.F Snieziko, ed. A symposium on disease of fishes and shellfishes, American Fisheries Society, Special Publication 5.Bethesda, pp.21-29.
Musa,N., Wei, L.S. and Wendy, W. (2009): Bacterial diseases outbreak of African Catfish (Clarias gariepinus) from Manir River, Terengganu, Malaysia. J lif. Sci.,volume 3 no (5) :10-20.
Nadheer A. Fazaa Jonathon C. Dunn & Mark J. Whittingham 2015. Status of Euphrates Soft-shelled Turtle Rafetus euphraticus in the Iraqi Central Marsh. International Conference on Latest Trends in Food, Biological & Ecological Sciences (ICLTFBE'15) Oct. 11-12, 2015 Dubai (UAE)
Newell DG, Koopmans M, Verhoef L, Duizer E, Aidara-Kane A, Sprong H, Opsteegh M, Langelaar M, Threfall J, Scheutz F, van der Giessen J, Kruse H. 2010. Food-borne Diseases—the Challenges of 20 Years Ago Still Persist While New Ones Continue to Emerge. International Journal of Food Microbiology. 139(Suppl. 1):S3–S15.
Newell DG, Koopmans M, Verhoef L, Duizer E, Aidara-Kane A, Sprong H, Opsteegh M, Langelaar M, Threfall J, Scheutz F, van der Giessen J, Kruse H. 2010. Food-borne Diseases—the Challenges of 20 Years Ago Still Persist While New Ones Continue to Emerge. International Journal of Food Microbiology. 139(Suppl. 1):S3–S15.
Nils kautsky, Patrik Romback, Michael Tedengren and Max Troell. 2000. Ecosystem perspectives on management of disease in shrimp pond farming. Aquaculture19: 145-161.
Oni, T. A. , Olaleye, V.F. and Omafuvbe, B.O (2013): Preliminary studies on associated bacterial and fungal load of artificially cultured Clarias gariepinus (Burchell,1822) fingerlings. Iife Journal of Science vol. 15, no. 1 (2013).
Pelczar MJ, Bard RC, Burnett GW, Conn HJ, Demoss RD, Euans EE, Weiss FA, Jennison MW, Meckee AP, Riker AJ, Warren J, Weeks OB (1957). Manual of microbiological methods. Society of American Bacteriology. McGraw Hill Book Company, Inc., New York, p. 315.
Real L. A., 1996, Sustainability and ecology of infectious diseases, Bioscience, 46: 88-97, http://dx.doi.org/10.2307/1312811
Roberts, R. J. (2001) “Fish pathology” 3rd Edition, 2001. Bailliere tindall, London England
Roberts, R. J. and Horne, M. T. (1978): Bacterial meningitis in farmed rainbow trout Salmo gairdneri Tichardison, affected with chronic pancreatic necrosis. J. of Fish Diseases. 1, 157 – 164.
Smith JL, Fratamico PM, Gunther NW. 2007. Extraintestinal Pathogenic Escherichia coli. Foodborne Pathogenic Diseases. 4:134-163.
Sneath PHA (1986). Endospore-forming Gram-positive rods and cocci. In: (Ed. Sneath, PHA). William and Wilkins, Baltimore, Bergey's Manual Syst. Bacteriol. 2: 141-219.
Somsiri, T. and Soontornvit, S. 2002. Bacterial diseases of cultured tiger frog (Rana tigerina). In C.R. LavillaPitogo and E.R. Cruz-Lacierda (eds.), Diseases in Asian Aquaculture IV, Fish Health Section, Asian Fisheries Society, Manila.
T, Jamal M, Nighat F, Andleeb S. 2014. Broad Spectrum Antibiotics and Resistance in Non-target Bacteria: An Example from Tetracycline. Journal of Pure and Applied Microbiology. 8(4): 2667-2671.
Von BH, Marre R. 2005. Antimicrobial resistance of Escherichia coli and therapeutic implications. International Journal of Medical Microbiology. 295: 503–11.
Wakabayashi H and Egusa S (1972) Characteristics of a Pseudomonas sp.
from an epizootic of pond-cultured eels (Anguilla japonica). Bulletin of the Japanese Society of Scientific Fisheries 38 (6): 577-587.
© 2017 The Author(s). Published by All-Russian Scientific Research Institute of Fundamental and Applied Parasitology of Animals and Plants named after K.I. Skryabin. This is an open access article under the Agreement of 02.07.2014 (Russian Science Citation Index (RSCI) http://elibrary.ru/projects/citation/cit_index.asp) and the Agreement of 12.06.2014 (CA-BI.org/Human Sciences section: http://www.cabi.org/Uploads/CABI/publishing/fulltext-products/cabi-fulltext-material-from-journals-b...)